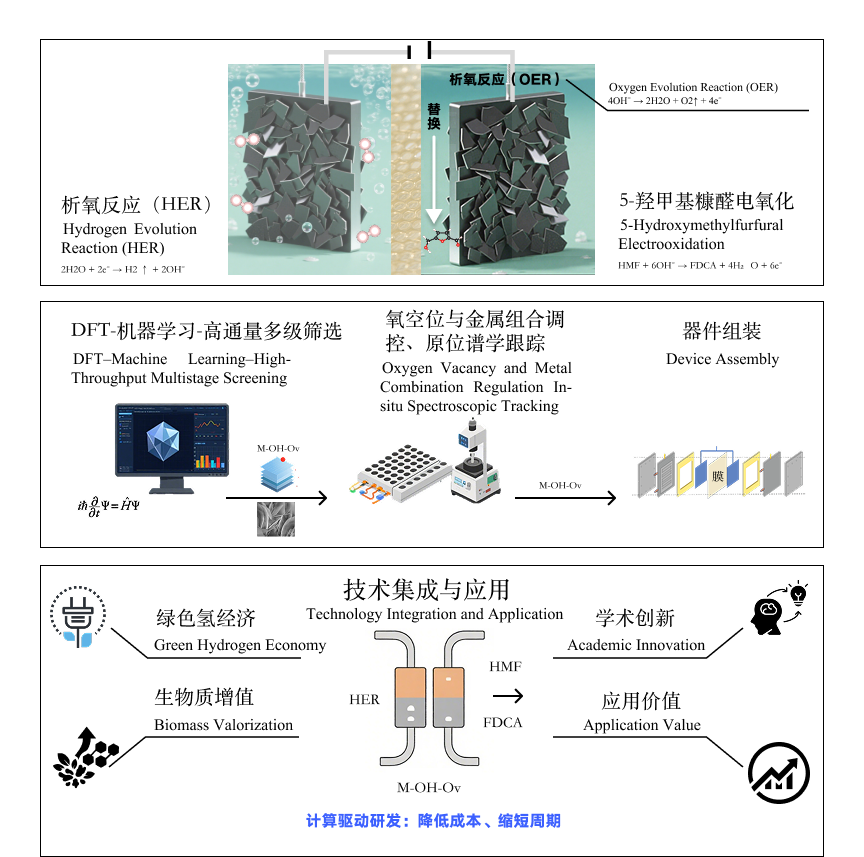
Developing integrated technologies for low-energy-consumption water electrolysis hydrogen production and biomass valorization is of great significance for achieving the carbon peaking and carbon neutrality goals. This research aims to construct a synergistic system for low - energy hydrogen production and biomass valorization by coupling the alkaline hydrogen evolution reaction at the cathode with the electrochemical oxidation of 5-hydroxymethylfurfural (HMF) to 2,5-furandicarboxylic acid (FDCA) at the anode. Addressing the bottlenecks of high cost and insufficient bifunctional activity of existing electrode materials, this project proposes a “computational - experimental” collaborative strategy: through a three-tier strategy combining initial DFT screening, machine learning prediction, and high-throughput validation, non-noble metal layered hydroxides (M-OH-Ov) modified with oxygen vacancies will be constructed. The project will systematically elucidate the regulatory effects of metal components and oxygen vacancy concentration on the geometric/electronic structure of active sites. Combining in-situ characterization and microkinetic modeling, the atomic-level evolution mechanisms of anode carboxyl group formation and cathode O - H bond activation will be clarified, establishing a quantitative structure-activity relationship between structural parameters (e.g., valence electron number) and catalytic performance (e.g., activity, selectivity). On this basis, bifunctional electrodes will be developed to integrate water electrolysis with biomass conversion. By leveraging computation-driven high-throughput screening to overcome the limitations of traditional trial-and-error methods, the project will reduce R&D costs and provide core independent technologies for the green hydrogen economy and biomass resource utilization, demonstrating both academic innovation and application value.
Date: September 1st, 2025
Issued by: School of Materials Science and Engineering